Fish
| Fish Temporal range: Middle Cambrian – Recent
| |
|---|---|

| |
| Bala shark, a bony fish | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Olfactores |
| Subphylum: | Vertebrata |
| Groups included | |
| Cladistically included but traditionally excluded taxa | |
A fish (pl.: fish or fishes) is an aquatic, anamniotic, gill-bearing vertebrate animal with swimming fins and a hard skull, but lacking limbs with digits. Fish can be grouped into the more basal jawless fish and the more common jawed fish, the latter including all living cartilaginous and bony fish, as well as the extinct placoderms and acanthodians. Most fish are cold-blooded, their body temperature varying with the surrounding water, though some large active swimmers like white shark and tuna can hold a higher core temperature. Many fish can communicate acoustically with each other, such as during courtship displays.
The earliest fish appeared during the Cambrian as small filter feeders; they continued to evolve through the Paleozoic, diversifying into many forms. The earliest fish with dedicated respiratory gills and paired fins, the ostracoderms, had heavy bony plates that served as protective exoskeletons against invertebrate predators. The first fish with jaws, the placoderms, appeared in the Silurian and greatly diversified during the Devonian, the "Age of Fishes".
Bony fish, distinguished by the presence of swim bladders and later ossified endoskeletons, emerged as the dominant group of fish after the end-Devonian extinction wiped out the apex placoderms. Bony fish are further divided into the lobe-finned and ray-finned fish. About 96% of all living fish species today are teleosts, a crown group of ray-finned fish that can protrude their jaws. The tetrapods, a mostly terrestrial clade of vertebrates that have dominated the top trophic levels in both aquatic and terrestrial ecosystems since the Late Paleozoic, evolved from lobe-finned fish during the Carboniferous, developing air-breathing lungs homologous to swim bladders. Despite the cladistic lineage, tetrapods are usually not considered to be fish, making "fish" a paraphyletic group.
Fish have been an important natural resource for humans since prehistoric times, especially as food. Commercial and subsistence fishers harvest fish in wild fisheries or farm them in ponds or in breeding cages in the ocean. Fish are caught for recreation, or raised by fishkeepers as ornaments for private and public exhibition in aquaria and garden ponds. Fish have had a role in human culture through the ages, serving as deities, religious symbols, and as the subjects of art, books and movies.
Etymology
The word fish is inherited from Proto-Germanic, and is related to German Fisch, the Latin piscis and Old Irish īasc, though the exact root is unknown; some authorities reconstruct a Proto-Indo-European root *peysk-, attested only in Italic, Celtic, and Germanic.[1][2][3][4]
Evolution
Fossil history

About 530 million years ago during the Cambrian explosion, fishlike animals with a notochord and eyes at the front of the body, such as Haikouichthys, appear in the fossil record.[5] During the late Cambrian, other jawless forms such as conodonts appear.[6][7]
Jawed vertebrates appear in the Silurian, with giant armoured placoderms such as Dunkleosteus.[8] Jawed fish, too, appeared during the Silurian:[9] the cartilaginous Chondrichthyes[10][11] and the bony Osteichthyes.[12]
During the Devonian, fish diversity greatly increased, including among the placoderms, lobe-finned fishes, and early sharks, earning the Devonian the epithet "the age of fishes".[13][14]
Phylogeny
Fishes are a paraphyletic group, since any clade containing all fish, such as the Gnathostomata or (for bony fish) Osteichthyes, also contains the clade of tetrapods (four-limbed vertebrates, mostly terrestrial), which are usually not considered fish.[15][16] Some tetrapods, such as cetaceans and ichthyosaurs, have secondarily acquired a fish-like body shape through convergent evolution.[17] Fishes of the World comments that "it is increasingly widely accepted that tetrapods, including ourselves, are simply modified bony fishes, and so we are comfortable with using the taxon Osteichthyes as a clade, which now includes all tetrapods".[16] The biodiversity of extant fish is unevenly distributed among the various groups; teleosts, bony fishes able to protrude their jaws, make up 96% of fish species.[18][16] The cladogram[19] shows the evolutionary relationships of all groups of living fishes (with their respective diversity[16]) and the tetrapods.[20] Extinct groups are marked with a dagger (†); groups of uncertain placement[19] are labelled with a question mark (?) and dashed lines (- - - - -).
| Vertebrates |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Taxonomy
Fishes (without tetrapods) are a paraphyletic group and for this reason, the class Pisces seen in older reference works is no longer used in formal classifications. Traditional classification divides fish into three extant classes (Agnatha, Chondrichthyes, and Osteichthyes), and with extinct forms sometimes classified within those groups, sometimes as their own classes.[21]
Fish account for more than half of vertebrate species. As of 2016, there are over 32,000 described species of bony fish, over 1,100 species of cartilaginous fish, and over 100 hagfish and lampreys. A third of these fall within the nine largest families; from largest to smallest, these are Cyprinidae, Gobiidae, Cichlidae, Characidae, Loricariidae, Balitoridae, Serranidae, Labridae, and Scorpaenidae. About 64 families are monotypic, containing only one species.[16]
Diversity
Fish range in size from the huge 16-metre (52 ft) whale shark[22] to some tiny teleosts only 8-millimetre (0.3 in) long, such as the cyprinid Paedocypris progenetica[23] and the stout infantfish.[24]
-
Largest: whale shark
-
Smallest: e.g. P. progenetica
Swimming performance varies from fish such as tuna, salmon, and jacks that can cover 10–20 body-lengths per second to species such as eels and rays that swim no more than 0.5 body-lengths per second.[25]
A typical fish is cold-blooded, has a streamlined body for rapid swimming, extracts oxygen from water using gills, has two sets of paired fins, one or two dorsal fins, an anal fin and a tail fin, jaws, skin covered with scales, and lays eggs. Each criterion has exceptions, creating a wide diversity in body shape and way of life. For example, some fast-swimming fish are warm-blooded, while some slow-swimming fish have abandoned streamlining in favour of other body shapes.[26]
Ecology
Habitats
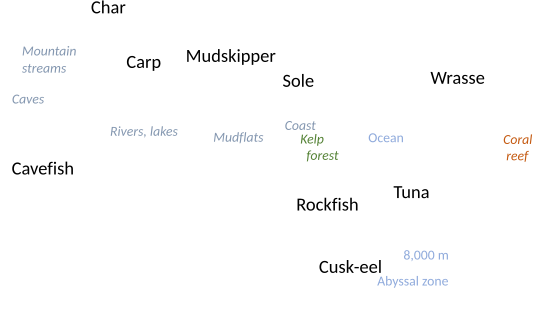
Fish species are roughly divided equally between freshwater and marine (oceanic) ecosystems; there are some 15,200 freshwater species and around 14,800 marine species.[27] Coral reefs in the Indo-Pacific constitute the center of diversity for marine fishes,[28] whereas continental freshwater fishes are most diverse in large river basins of tropical rainforests, especially the Amazon, Congo, and Mekong basins.[29] More than 5,600 fish species inhabit Neotropical freshwaters alone, such that Neotropical fishes represent about 10% of all vertebrate species on the Earth.[30]
Fish are abundant in most bodies of water. They can be found in nearly all aquatic environments, from high mountain streams (e.g., char and gudgeon) to the abyssal and even hadal depths of the deepest oceans (e.g., cusk-eels and snailfish), although none have been found in the deepest 25% of the ocean.[31] The deepest living fish in the ocean so far found is a cusk-eel, Abyssobrotula galatheae, recorded at the bottom of the Puerto Rico trench at 8,370 m (27,460 ft).[32]
In terms of temperature, Jonah's icefish live in cold[a] waters of the Southern Ocean, including under the Filchner–Ronne Ice Shelf at a latitude of 79°S,[34] while desert pupfish live in desert springs, streams, and marshes, sometimes highly saline, with water temperatures as high as 36 C.[35][36]
A few fish live mostly on land or lay their eggs on land near water.[37] Mudskippers feed and interact with one another on mudflats and go underwater to hide in their burrows.[38] A single undescribed species of Phreatobius has been called a true "land fish" as this worm-like catfish strictly lives among waterlogged leaf litter.[39][40] Cavefish of multiple families live in underground lakes, underground rivers or aquifers.[41]
Parasites and predators
Like other animals, fish suffer from parasitism. Some species use cleaner fish to remove external parasites. The best known of these are the bluestreak cleaner wrasses of coral reefs in the Indian and Pacific oceans. These small fish maintain cleaning stations where other fish congregate and perform specific movements to attract the attention of the cleaners.[42] Cleaning behaviors have been observed in a number of fish groups, including an interesting case between two cichlids of the same genus, Etroplus maculatus, the cleaner, and the much larger E. suratensis.[43]
Fish occupy many trophic levels in freshwater and marine food webs. Fish at the higher levels are predatory, and a substantial part of their prey consists of other fish.[44] In addition, mammals such as dolphins and seals feed on fish, alongside birds such as gannets and cormorants.[45]
Anatomy and physiology
Anatomy and locomotion
The body of a typical fish is adapted for efficient swimming by alternately contracting paired sets of muscles on either side of the backbone. These contractions form S-shaped curves that move down the body. As each curve reaches the tail fin, force is applied to the water, moving the fish forward. The other fins act as control surfaces like an aircraft's flaps, enabling the fish to steer in any direction.[46]
-
Anatomy of a typical fish (lanternfish shown):
1) gill cover 2) lateral line 3) dorsal fin 4) fat fin
5) caudal peduncle 6) caudal fin 7) anal fin 8) photophores 9) pelvic fins 10) pectoral fins
Since body tissue is denser than water, fish must compensate for the difference or they will sink. Many bony fish have an internal organ called a swim bladder that allows them to adjust their buoyancy by increasing or decreasing the amount of gas it contains.[47]
The scales of fish provide protection from predators at the cost of adding stiffness and weight.[48] Fish scales are often highly reflective; this silvering provides camouflage in the open ocean. Because the water all around is the same colour, reflecting an image of the water offers near-invisibility.[49]
Circulation
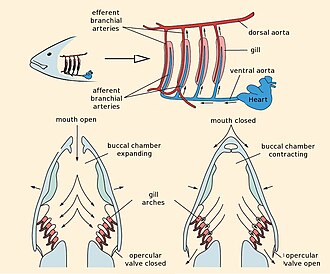
Fish have a closed-loop circulatory system. The heart pumps the blood in a single loop throughout the body; for comparison, the mammal heart has two loops, one for the lungs to pick up oxygen, one for the body to deliver the oxygen. In fish, the heart pumps blood through the gills. Oxygen-rich blood then flows without further pumping, unlike in mammals, to the body tissues. Finally, oxygen-depleted blood returns to the heart.[50]
Respiration
Gills
Fish exchange gases using gills on either side of the pharynx. Gills consist of comblike structures called filaments. Each filament contains a capillary network that provides a large surface area for exchanging oxygen and carbon dioxide. Fish exchange gases by pulling oxygen-rich water through their mouths and pumping it over their gills. Capillary blood in the gills flows in the opposite direction to the water, resulting in efficient countercurrent exchange. The gills push the oxygen-poor water out through openings in the sides of the pharynx. Cartilaginous fish have multiple gill openings: sharks usually have five, sometimes six or seven pairs; they often have to swim to oxygenate their gills. Bony fish have a single gill opening on each side, hidden beneath a protective bony cover or operculum. They are able to oxygenate their gills using muscles in the head.[51]
Air breathing
Some 400 species of fish in 50 families can breathe air, enabling them to live in oxygen-poor water or to emerge on to land.[52] The ability of fish to do this is potentially limited by their single-loop circulation, as oxygenated blood from their air-breathing organ will mix with deoxygenated blood returning to the heart from the rest of the body. Lungfish, bichirs, ropefish, bowfins, snakefish, and the African knifefish have evolved to reduce such mixing, and to reduce oxygen loss from the gills to oxygen-poor water. Bichirs and lungfish have tetrapod-like paired lungs, requiring them to surface to gulp air, and making them obligate air breathers. Many other fish, including inhabitants of rock pools and the intertidal zone, are facultative air breathers, able to breathe air when out of water, as may occur daily at low tide, and to use their gills when in water. Some coastal fish like rockskippers and mudskippers choose to leave the water to feed in habitats temporarily exposed to the air.[52] Some catfish absorb air through their digestive tracts.[53]
Digestion
The digestive system consists of a tube, the gut, leading from the mouth to the anus. The mouth of most fishes contains teeth to grip prey, bite off or scrape plant material, or crush the food. An esophagus carries food to the stomach where it may be stored and partially digested. A sphincter, the pylorus, releases food to the intestine at intervals. Many fish have finger-shaped pouches, pyloric caeca, around the pylorus, of doubtful function. The pancreas secretes enzymes into the intestine to digest the food; other enzymes are secreted directly by the intestine itself. The liver produces bile which helps to break up fat into an emulsion which can be absorbed in the intestine.[54]
Excretion
Most fish release their nitrogenous wastes as ammonia. This may be excreted through the gills or filtered by the kidneys. Salt is excreted by the rectal gland.[55] Saltwater fish tend to lose water by osmosis; their kidneys return water to the body, and produce a concentrated urine. The reverse happens in freshwater fish: they tend to gain water osmotically, and produce a dilute urine. Some fish have kidneys able to operate in both freshwater and saltwater.[56]
Brain

Fish have small brains relative to body size compared with other vertebrates, typically one-fifteenth the brain mass of a similarly sized bird or mammal.[57] However, some fish have relatively large brains, notably mormyrids and sharks, which have brains about as large for their body weight as birds and marsupials.[58] At the front of the brain are the olfactory lobes, a pair of structures that receive and process signals from the nostrils via the two olfactory nerves. Fish that hunt primarily by smell, such as hagfish and sharks, have very large olfactory lobes. Behind these is the telencephalon, which in fish deals mostly with olfaction. Together these structures form the forebrain. Connecting the forebrain to the midbrain is the diencephalon; it works with hormones and homeostasis. The pineal body is just above the diencephalon; it detects light, maintains circadian rhythms, and controls color changes. The midbrain contains the two optic lobes. These are very large in species that hunt by sight, such as rainbow trout and cichlids. The hindbrain controls swimming and balance.The single-lobed cerebellum is the biggest part of the brain; it is small in hagfish and lampreys, but very large in mormyrids, processing their electrical sense. The brain stem or myelencephalon controls some muscles and body organs, and governs respiration and osmoregulation.[57]
Sensory systems
The lateral line system is a network of sensors in the skin which detects gentle currents and vibrations, and senses the motion of nearby fish, whether predators or prey.[59] This can be considered both a sense of touch and of hearing. Blind cave fish navigate almost entirely through the sensations from their lateral line system.[60] Some fish, such as catfish and sharks, have the ampullae of Lorenzini, electroreceptors that detect weak electric currents on the order of millivolt.[61]
Vision is an important sensory system in fish.[62] Fish eyes are similar to those of terrestrial vertebrates like birds and mammals, but have a more spherical lens.[62] Their retinas generally have both rods and cones (for scotopic and photopic vision); many species have colour vision, often with three types of cone.[62] Teleosts can see polarized light;[63] some such as cyprinids have a fourth type of cone that detects ultraviolet.[62] Amongst jawless fish, the lamprey has well-developed eyes,[64] while the hagfish has only primitive eyespots.[65]
Hearing too is an important sensory system in fish. Fish sense sound using their lateral lines and otoliths in their ears, inside their heads. Some can detect sound through the swim bladder.[66]
Some fish, including salmon, are capable of magnetoreception; when the axis of a magnetic field is changed around a circular tank of young fish, they reorient themselves in line with the field.[67][68] The mechanism of fish magnetoreception remains unknown;[69] experiments in birds imply a quantum radical pair mechanism.[70]
Cognition
The cognitive capacities of fish include self-awareness, as seen in mirror tests. Manta rays and wrasses placed in front of a mirror repeatedly check whether their reflection's behavior mimics their body movement.[71][72] Choerodon wrasse, archerfish, and Atlantic cod can solve problems and invent tools.[73] The monogamous cichlid Amatitlania siquia exhibits pessimistic behavior when prevented from being with its partner.[74] Fish orient themselves using landmarks; they may use mental maps based on multiple landmarks. Fish are able to learn to traverse mazes, showing that they possess spatial memory and visual discrimination.[75] Behavioral research suggests that fish are sentient, capable of experiencing pain.[76]
Electrogenesis

Electric fish such as elephantfishes, the African knifefish, and electric eels have some of their muscles adapted to generate electric fields. They use the field to locate and identify objects such as prey in the waters around them, which may be turbid or dark.[61] Strongly electric fish like the electric eel can in addition use their electric organs to generate shocks powerful enough to stun their prey.[78]
Endothermy
Most fish are exclusively cold-blooded or ectothermic. However, the Scombroidei are warm-blooded (endothermic), including the billfishes and tunas.[79] The opah, a lampriform, uses whole-body endothermy, generating heat with its swimming muscles to warm its body while countercurrent exchange minimizes heat loss.[80] Among the cartilaginous fishes, sharks of the families Lamnidae (such as the great white shark) and Alopiidae (thresher sharks) are endothermic. The degree of endothermy varies from the billfishes, which warm only their eyes and brain, to the bluefin tuna and the porbeagle shark, which maintain body temperatures more than 20 °C (68 °F) above the ambient water.[79][81][82]
Reproduction and life-cycle
The primary reproductive organs are paired testicles and ovaries.[83] Eggs are released from the ovary to the oviducts.[84] Over 97% of fish, including salmon and goldfish, are oviparous, meaning that the eggs are shed into the water and develop outside the mother's body.[85] The eggs are usually fertilized outside the mother's body, with the male and female fish shedding their gametes into the surrounding water. In a few oviparous fish, such as the skates, fertilization is internal: the male uses an intromittent organ to deliver sperm into the female's genital opening of the female.[86] Marine fish release large numbers of small eggs into the open water column. Newly hatched young of oviparous fish are planktonic larvae. They have a large yolk sac and do not resemble juvenile or adult fish. The larval period in oviparous fish is usually only some weeks, and larvae rapidly grow and change in structure to become juveniles. During this transition, larvae must switch from their yolk sac to feeding on zooplankton prey.[86] Some fish such as surf-perches, splitfins, and lemon sharks are viviparous or live-bearing, meaning that the mother retains the eggs and nourishes the embryos via a structure analogous to the placenta to connect the mother's blood supply with the embryo's.[86]
DNA repair
Embryos of externally fertilized fish species are directly exposed during their development to environmental conditions that may damage their DNA, such as pollutants, UV light and reactive oxygen species.[87] To deal with such DNA damages, a variety of different DNA repair pathways are employed by fish embryos during their development.[87] In recent years zebrafish have become a useful model for assessing environmental pollutants that might be genotoxic, i.e. cause DNA damage.[88]
Defenses against disease
Fish have both non-specific and immune defenses against disease. Non-specific defenses include the skin and scales, as well as the mucus layer secreted by the epidermis that traps and inhibits the growth of microorganisms. If pathogens breach these defenses, the innate immune system can mount an inflammatory response that increases blood flow to the infected region and delivers white blood cells that attempt to destroy pathogens, non-specifically. Specific defenses respond to particular antigens, such as proteins on the surfaces of pathogenic bacteria, recognised by the adaptive immune system.[89] Immune systems evolved in deuterostomes as shown in the cladogram.[90]
Immune organs vary by type of fish. The jawless fish have lymphoid tissue within the anterior kidney, and granulocytes in the gut. They have their own type of adaptive immune system; it makes use of variable lymphocyte receptors (VLR) to generate immunity to a wide range of antigens, The result is much like that of jawed fishes and tetrapods, but it may have evolved separately.[90] All jawed fishes have an adaptive immune system with B and T lymphocytes bearing immunoglobulins and T cell receptors respectively. This makes use of Variable–Diversity–Joining rearrangement (V(D)J) to create immunity to a wide range of antigens. This system evolved once and is basal to the jawed vertebrate clade.[90] Cartilaginous fish have three specialized organs that contain immune system cells: the epigonal organs around the gonads, Leydig's organ within the esophagus, and a spiral valve in their intestine, while their thymus and spleen have similar functions to those of the same organs in the immune systems of tetrapods.[91] Teleosts have lymphocytes in the thymus, and other immune cells in the spleen and other organs.[92][93]
Behavior
Shoaling and schooling

A shoal is a loosely organised group where each fish swims and forages independently but is attracted to other members of the group and adjusts its behaviour, such as swimming speed, so that it remains close to the other members of the group. A school is a much more tightly organised group, synchronising its swimming so that all fish move at the same speed and in the same direction.[95] Schooling is sometimes an antipredator adaptation, offering improved vigilance against predators. It is often more efficient to gather food by working as a group, and individual fish optimise their strategies by choosing to join or leave a shoal. When a predator has been noticed, prey fish respond defensively, resulting in collective shoal behaviours such as synchronised movements. Responses do not consist only of attempting to hide or flee; antipredator tactics include for example scattering and reassembling. Fish also aggregate in shoals to spawn.[94] The capelin migrates annually in large schools between its feeding areas and its spawning grounds.[96]
Communication
Fish communicate by transmitting acoustic signals (sounds) to each other. This is most often in the context of feeding, aggression or courtship.[97] The sounds emitted vary with the species and stimulus involved. Fish can produce either stridulatory sounds by moving components of the skeletal system, or can produce non-stridulatory sounds by manipulating specialized organs such as the swimbladder.[98]

Some fish produce sounds by rubbing or grinding their bones together. These sounds are stridulatory. In Haemulon flavolineatum, the French grunt fish, as it produces a grunting noise by grinding its teeth together, especially when in distress. The grunts are at a frequency of around 700 Hz, and last approximately 47 milliseconds.[98] The longsnout seahorse, Hippocampus reidi produces two categories of sounds, 'clicks' and 'growls', by rubbing their coronet bone across the grooved section of their neurocranium.[99] Clicks are produced during courtship and feeding, and the frequencies of clicks were within the range of 50 Hz-800 Hz. The frequencies are at the higher end of the range during spawning, when the female and male fishes were less than fifteen centimeters apart. Growls are produced when the H. reidi are stressed. The 'growl' sounds consist of a series of sound pulses and are emitted simultaneously with body vibrations.[100]
Some fish species create noise by engaging specialized muscles that contract and cause swimbladder vibrations. Oyster toadfish produce loud grunts by contracting sonic muscles along the sides of the swim bladder.[101] Female and male toadfishes emit short-duration grunts, often as a fright response.[102] In addition to short-duration grunts, male toadfishes produce "boat whistle calls".[103] These calls are longer in duration, lower in frequency, and are primarily used to attract mates.[103] The various sounds have frequency range of 140 Hz to 260 Hz.[103] The frequencies of the calls depend on the rate at which the sonic muscles contract.[104][101]
The red drum, Sciaenops ocellatus, produces drumming sounds by vibrating its swimbladder. Vibrations are caused by the rapid contraction of sonic muscles that surround the dorsal aspect of the swimbladder. These vibrations result in repeated sounds with frequencies from 100 to >200 Hz. S. ocellatus produces different calls depending on the stimuli involved, such as courtship or a predator's attack. Females do not produce sounds, and lack sound-producing (sonic) muscles.[105]
Conservation
The 2024 IUCN Red List names 2,168 fish species that are endangered or critically endangered.[106] Included are species such as Atlantic cod,[107] Devil's Hole pupfish,[108] coelacanths,[109] and great white sharks.[110] Because fish live underwater they are more difficult to study than terrestrial animals and plants, and information about fish populations is often lacking. However, freshwater fish seem particularly threatened because they often live in relatively small water bodies. For example, the Devil's Hole pupfish occupies only a single 3 by 6 metres (10 by 20 ft) pool.[111]
Overfishing
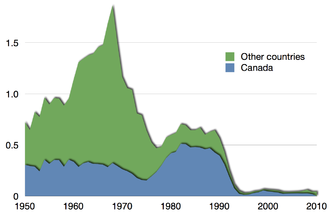
The Food and Agriculture Organization reports that "in 2017, 34 percent of the fish stocks of the world's marine fisheries were classified as overfished".[113] Overfishing is a major threat to edible fish such as cod and tuna.[114][115] Overfishing eventually causes fish stocks to collapse, because the survivors cannot produce enough young to replace those removed. Such commercial extinction does not mean that the species is extinct, merely that it can no longer sustain a fishery. In the case of the Pacific sardine fishery off the California coast, the catch steadily declined from a 1937 peak of 800,000 tonnes to an economically inviable 24,000 tonnes in 1968.[116] In the case of the Atlantic northwest cod fishery, overfishing reduced the fish population to 1% of its historical level by 1992.[112] Fisheries scientists and the fishing industry have sharply differing views on the resiliency of fisheries to intensive fishing. In many coastal regions the fishing industry is a major employer, so governments are predisposed to support it.[117][118] On the other hand, scientists and conservationists push for stringent protection, warning that many stocks could be destroyed within fifty years.[119][120]
Other threats
A key stress on both freshwater and marine ecosystems is habitat degradation including water pollution, the building of dams, removal of water for use by humans, and the introduction of exotic species including predators.[121] Freshwater fish, especially if endemic to a region (occurring nowhere else), may be threatened with extinction for all these reasons, as is the case for three of Spain's ten endemic freshwater fishes.[122] River dams, especially major schemes like the Kariba Dam (Zambezi river) and the Aswan Dam (River Nile) on rivers with economically important fisheries, have caused large reductions in fish catch.[123] Industrial bottom trawling can damage seabed habitats, as has occurred on the Georges Bank in the North Atlantic.[124] Introduction of aquatic invasive species is widespread. It modifies ecosystems, causing biodiversity loss, and can harm fisheries. Harmful species include fish but are not limited to them;[125] the arrival of a comb jelly in the Black Sea damaged the anchovy fishery there.[126][125] The opening of the Suez Canal in 1869 made possible Lessepsian migration, facilitating the arrival of hundreds of Indo-Pacific marine species of fish, algae and invertebrates in the Mediterranean Sea, deeply impacting its overall biodiversity [127] and ecology.[128] The predatory Nile perch was deliberately introduced to Lake Victoria in the 1960s as a commercial and sports fish. The lake had high biodiversity, with some 500 endemic species of cichlid fish. It drastically altered the lake's ecology, and simplified the fishery from multi-species to just three: the Nile perch, the silver cyprinid, and another introduced fish, the Nile tilapia. The haplochromine cichlid populations have collapsed.[129][130]
Importance to humans
Economic

Throughout history, humans have used fish as a food source for dietary protein. Historically and today, most fish harvested for human consumption has come by means of catching wild fish. However, fish farming, which has been practiced since about 3,500 BCE in ancient China,[131] is becoming increasingly important in many nations. Overall, about one-sixth of the world's protein is estimated to be provided by fish.[132] Fishing is accordingly a large global business which provides income for millions of people.[132] The Environmental Defense Fund has a guide on which fish are safe to eat, given the state of pollution in today's world, and which fish are obtained in a sustainable way.[133] As of 2020, over 65 million tonnes (Mt) of marine fish and 10 Mt of freshwater fish were captured, while some 50 Mt of fish, mainly freshwater, were farmed. Of the marine species captured in 2020, anchoveta represented 4.9 Mt, Alaska pollock 3.5 Mt, skipjack tuna 2.8 Mt, and Atlantic herring and yellowfin tuna 1.6 Mt each; eight more species had catches over 1 Mt.[134]
Recreation
Fish have been recognized as a source of beauty for almost as long as used for food, appearing in cave art, being raised as ornamental fish in ponds, and displayed in aquariums in homes, offices, or public settings. Recreational fishing is fishing primarily for pleasure or competition; it can be contrasted with commercial fishing, which is fishing for profit, or artisanal fishing, which is fishing primarily for food. The most common form of recreational fishing employs a rod, reel, line, hooks, and a wide range of baits. Recreational fishing is particularly popular in North America and Europe; government agencies often actively manage target fish species.[135][136]
Culture
Fish themes have symbolic significance in many religions. In ancient Mesopotamia, fish offerings were made to the gods from the very earliest times.[137] Fish were also a major symbol of Enki, the god of water.[137] Fish frequently appear as filling motifs in cylinder seals from the Old Babylonian (c. 1830 BC – c. 1531 BC) and Neo-Assyrian (911–609 BC) periods.[137] Starting during the Kassite Period (c. 1600 BC – c. 1155 BC) and lasting until the early Persian Period (550–30 BC), healers and exorcists dressed in ritual garb resembling the bodies of fish.[137] During the Seleucid Period (312–63 BC), the legendary Babylonian culture hero Oannes was said to have dressed in the skin of a fish.[137] Fish were sacred to the Syrian goddess Atargatis[138] and, during her festivals, only her priests were permitted to eat them.[138] In the Book of Jonah, the central figure, a prophet named Jonah, is swallowed by a giant fish after being thrown overboard by the crew of the ship he is travelling on.[139] Early Christians used the ichthys, a symbol of a fish, to represent Jesus.[138][140] Among the deities said to take the form of a fish are Ikatere of the Polynesians,[141] the shark-god Kāmohoaliʻi of Hawaiʻi,[142] and Matsya of the Hindus.[143] The constellation Pisces ("The Fishes") is associated with a legend from Ancient Rome that Venus and her son Cupid were rescued by two fishes.[144]
Fish feature prominently in art,[145] in films such as Finding Nemo[146] and books such as The Old Man and the Sea.[147] Large fish, particularly sharks, have frequently been the subject of horror movies and thrillers, notably the novel Jaws, made into a film which in turn has been parodied and imitated many times.[148] Piranhas are shown in a similar light to sharks in films such as Piranha.[149]
-
The Fishmonger's Shop, Bartolomeo Passerotti, 1580s
-
Goldfish by Henri Matisse, 1912
See also
- Deep sea fish
- Fish acute toxicity syndrome
- Fish development
- Forage fish
- Ichthyology
- List of fish common names
- List of fish families
- Mercury in fish
- Otolith – bone used for determining the age of a fish
- Pregnancy (fish)
- Walking fish
Notes
- ^ The temperature is often around 0 C. The freezing point of seawater at the surface is -1.85 C, falling to -2.62 C at a depth of 1000 metres. However, the water can be supercooled somewhat below these temperatures.[33]
References
- ^ "DWDS – Digitales Wörterbuch der deutschen Sprache". DWDS (in German). Archived from the original on 31 July 2020. Retrieved 21 January 2023.
- ^ Winfred Philipp Lehmann, Helen-Jo J. Hewitt, Sigmund Feist, A Gothic etymological dictionary, 1986, s.v. fisks p. 118
- ^ "fish, n.1". Oxford University Press. Archived from the original on 17 March 2023. Retrieved 21 January 2023.
- ^ Buck, Carl Darling (1949). "section 3.65". A Dictionary of Selected Synonyms in the Principal Indo-European Languages. p. 184.
- ^ Shu, D. G.; Conway Morris, S.; Han, J.; Zhang, Z. F.; Yasui, K.; Janvier, P.; et al. (2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature. 421 (6922): 526–529. Bibcode:2003Natur.421..526S. doi:10.1038/nature01264. PMID 12556891. S2CID 4401274.
- ^ Donoghue, Philip C. J.; Purnell, Mark A. (2009). "The Evolutionary Emergence of Vertebrates From Among Their Spineless Relatives". Evolution: Education and Outreach. 2 (2): 204–212. doi:10.1007/s12052-009-0134-3. ISSN 1936-6426.
- ^ Miller, James F.; Clark, D. L. (1984). "Cambrian and earliest Ordovician conodont evolution, biofacies, and provincialism". Geological Society of America Special Paper. Geological Society of America Special Papers. 196 (196): 43–68. doi:10.1130/SPE196-p43. ISBN 978-0-8137-2196-5.
- ^ "Monster fish crushed opposition with strongest bite ever". Smh.com.au. 30 November 2006. Archived from the original on 2 April 2013. Retrieved 26 February 2013.
- ^ Choo, Brian; Zhu, Min; Zhao, Wenjin; Jia, Liaotao; Zhu, You'an (2014). "The largest Silurian vertebrate and its palaeoecological implications". Scientific Reports. 4: 5242. Bibcode:2014NatSR...4.5242C. doi:10.1038/srep05242. PMC 4054400. PMID 24921626.
- ^ Andreev, Plamen S.; Sansom, Ivan J.; Li, Qiang; Zhao, Wenjin; Wang, Jianhua; Wang, Chun-Chieh; et al. (September 2022). "Spiny chondrichthyan from the lower Silurian of South China". Nature. 609 (7929): 969–974. Bibcode:2022Natur.609..969A. doi:10.1038/s41586-022-05233-8. PMID 36171377. S2CID 252570103.
- ^ Andreev, Plamen S.; Sansom, Ivan J.; Li, Qiang; Zhao, Wenjin; Wang, Jianhua; Wang, Chun-Chieh; et al. (September 2022). "The oldest gnathostome teeth". Nature. 609 (7929): 964–968. Bibcode:2022Natur.609..964A. doi:10.1038/s41586-022-05166-2. ISSN 0028-0836. PMID 36171375. S2CID 252569771.
- ^ Berg, Linda R.; Solomon, Eldra Pearl; Martin, Diana W. (2004). Biology. Cengage Learning. p. 599. ISBN 978-0-534-49276-2.
- ^ Benton 2005, p. 35: Fig 2.10, p. 73: Fig 3.25.
- ^ Dalton, Rex (January 2006). "Hooked on fossils". Nature. 439 (7074): 262–263. doi:10.1038/439262a. PMID 16421540. S2CID 4357313.
- ^ Greene, Harry W. (1 January 1998). "We are primates and we are fish: Teaching monophyletic organismal biology". Integrative Biology. 1 (3): 108–111. doi:10.1002/(sici)1520-6602(1998)1:3<108::aid-inbi5>3.0.co;2-t. ISSN 1520-6602.
- ^ a b c d e Nelson 2016, p. 3
- ^ Davis, R. W. (2019). "Return to the Sea: The Evolution of Marine Mammals". In Davis, R. W. (ed.). Marine Mammals: Adaptations for an Aquatic Life. New York: Springer International Publishing. pp. 7–27. ISBN 978-3-3199-8278-6.
- ^ Benton 2005, pp. 175–184.
- ^ a b Friedman, Matt; Sallan, Lauren Cole (June 2012). "Five hundred million years of extinction and recovery: A Phanerozoic survey of large-scale diversity patterns in fishes". Palaeontology. 55 (4): 707–742. Bibcode:2012Palgy..55..707F. doi:10.1111/j.1475-4983.2012.01165.x. S2CID 59423401.
- ^ "Summary Statistics". IUCN Red List of Threatened Species. 2023.1. Retrieved 5 February 2024. Table 1a: Number of species evaluated in relation to the overall number of described species, and numbers of threatened species by major groups of organisms
- ^ Benton, M.J. (1998). "The quality of the fossil record of vertebrates". In Donovan, S.K.; Paul, C.R.C. (eds.). The adequacy of the fossil record. Wiley. pp. 269–303, Fig. 2.
- ^ McClain, Craig R.; Balk, Meghan A.; Benfield, Mark C.; Branch, Trevor A.; Chen, Catherine; Cosgrove, James; et al. (13 January 2015). "Sizing ocean giants: patterns of intraspecific size variation in marine megafauna". PeerJ. 3: e715. doi:10.7717/peerj.715. ISSN 2167-8359. PMC 4304853. PMID 25649000.
- ^ Kottelat, Maurice; Britz, Ralf; Heok Hui, Tan; Witte, Kai-Erik (2005). "Paedocypris, a new genus of Southeast Asian cyprinid fish with a remarkable sexual dimorphism, comprises the world's smallest vertebrate" (PDF). Proceedings of the Royal Society B. 273 (1589): 895–899. doi:10.1098/rspb.2005.3419. PMC 1560243. PMID 16627273. Archived from the original (PDF) on 12 July 2009. Retrieved 26 October 2012.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Schindleria brevipinguis". FishBase. September 2017 version.
- ^ Helfman, Collette & Facey 1997, p. 103.
- ^ Helfman, Collette & Facey 1997, pp. 3, 33–36.
- ^ Manel, Stéphanie; Guerin, Pierre-Edouard; Mouillot, David; Blanchet, Simon; Velez, Laure; Albouy, Camille; Pellissier, Loïc (10 February 2020). "Global determinants of freshwater and marine fish genetic diversity". Nature Communications. 11 (1): 692. Bibcode:2020NatCo..11..692M. doi:10.1038/s41467-020-14409-7. ISSN 2041-1723. PMC 7010757. PMID 32041961.
- ^ Hubert, Nicolas; Meyer, Christopher P.; Bruggemann, Henrich J.; Guérin, Fabien; Komeno, Roberto J. L.; Espiau, Benoit; Causse, Romain; Williams, Jeffrey T.; Planes, Serge (15 March 2012). "Cryptic Diversity in Indo-Pacific Coral-Reef Fishes Revealed by DNA-Barcoding Provides New Support to the Centre-of-Overlap Hypothesis". PLOS ONE. 7 (3): e28987. Bibcode:2012PLoSO...728987H. doi:10.1371/journal.pone.0028987. ISSN 1932-6203. PMC 3305298. PMID 22438862.
- ^ van der Sleen, Peter; Albert, James S. (2022). "Patterns in Freshwater Fish Diversity". Encyclopedia of Inland Waters. Elsevier. pp. 243–255. doi:10.1016/b978-0-12-819166-8.00056-6. ISBN 978-0-12-822041-2.
- ^ Albert, James S.; Carvalho, Tiago P.; Petry, Paulo (June 2011). "Aquatic Biodiversity in the Amazon: Habitat Specialization and Geographic Isolation Promote Species Richness". Animals. 1 (2): 205–241. doi:10.3390/ani1020205. PMC 4513461. PMID 26486313.
- ^ Yancey, P.H.; Gerringer, M.E.; Drazen, J.C.; Rowden, A.A.; Jamieson, A. (2014). "Marine fish may be biochemically constrained from inhabiting the deepest ocean depths". Proc Natl Acad Sci USA. 111 (12): 4461–4465. Bibcode:2014PNAS..111.4461Y. doi:10.1073/pnas.1322003111. PMC 3970477. PMID 24591588.
- ^ "What is the deepest-living fish?". Australian Museum. 23 December 2014. Retrieved 18 September 2015.
- ^ Haumann, F. Alexander; Moorman, Ruth; Riser, Stephen C.; Smedsrud, Lars H.; Maksym, Ted; Wong, Annie P.S.; Wilson, Earle A.; Drucker, Robert; Talley, Lynne D.; Johnson, Kenneth S.; Key, Robert M.; Sarmiento, Jorge L. (28 October 2020). "Supercooled Southern Ocean Waters". Geophysical Research Letters. 47 (20). Bibcode:2020GeoRL..4790242H. doi:10.1029/2020GL090242. hdl:1912/26495.
- ^ Purser, Autun; Hehemann, Laura; Boehringer, Lilian; Tippenhauer, Sandra; Wege, Mia; Bornemann, Horst; et al. (2022). "A vast icefish breeding colony discovered in the Antarctic". Current Biology. 32 (4): 842–850.e4. Bibcode:2022CBio...32E.842P. doi:10.1016/j.cub.2021.12.022. hdl:2263/90796. PMID 35030328. S2CID 245936769.
- ^ Marsh, Paul C.; Sada, Donald W (1993). "Desert Pupfish (Cyprinodon macularius) Recovery Plan" (PDF). United States Fish and Wildlife Service. Archived (PDF) from the original on 17 October 2011.
- ^ Shrode, Joy B.; Gerking, Shelby D. (1977). "Effects of Constant and Fluctuating Temperatures on Reproductive Performance of a Desert Pupfish, Cyprinodon n. nevadensis". Physiological Zoology. 50 (1): 1–10. doi:10.1086/physzool.50.1.30155710. ISSN 0031-935X. S2CID 82166135.
- ^ Martin, K.L.M. (2014). Beach-Spawning Fishes: Reproduction in an Endangered Ecosystem. CRC Press. ISBN 978-1-4822-0797-2.
- ^ Froese, Rainer; Pauly, Daniel (eds.). "Periophthalmus barbarus". FishBase. November 2006 version.
- ^ "Cat-eLog: Heptapteridae: Phreatobius: Phreatobius sp. (1)". Planet Catfish. Archived from the original on 23 October 2006. Retrieved 26 November 2006.
- ^ Henderson, P.A.; Walker, I. (1990). "Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream". Journal of Fish Biology. 37 (3): 401–411. Bibcode:1990JFBio..37..401H. doi:10.1111/j.1095-8649.1990.tb05871.x.
- ^ Helfman, G.S. (2007). Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources. Island Press. pp. 41–42. ISBN 978-1-55963-595-0.
- ^ Helfman, Collette & Facey 1997, p. 380.
- ^ Wyman, Richard L.; Ward, Jack A. (1972). "A Cleaning Symbiosis between the Cichlid Fishes Etroplus maculatus and Etroplus suratensis. I. Description and Possible Evolution". Copeia. 1972 (4): 834–838. doi:10.2307/1442742. JSTOR 1442742.
- ^ Myers, Ransom A.; Worm, Boris (2003). "Rapid worldwide depletion of predatory fish communities". Nature. 423 (6937). Springer Science and Business Media: 280–283. Bibcode:2003Natur.423..280M. doi:10.1038/nature01610. ISSN 0028-0836. PMID 12748640.
- ^ "Predation". Northwest Power and Conservation Council. Retrieved 10 February 2024.
- ^ Sfakiotakis, M.; Lane, D. M.; Davies, J. B. C. (1999). "Review of Fish Swimming Modes for Aquatic Locomotion" (PDF). IEEE Journal of Oceanic Engineering. 24 (2): 237–252. Bibcode:1999IJOE...24..237S. doi:10.1109/48.757275. S2CID 17226211. Archived from the original (PDF) on 24 December 2013.
- ^ "Actinopterygii: More on Morphology". University of California Museum of Paleontology. Retrieved 10 February 2024.
- ^ Quan, Haocheng; Yang, Wen; Lapeyriere, Marine; Schaible, Eric; Ritchie, Robert O.; Meyers, Marc A. (2020). "Structure and Mechanical Adaptability of a Modern Elasmoid Fish Scale from the Common Carp". Matter. 3 (3): 842–863. doi:10.1016/j.matt.2020.05.011.
- ^ Herring, Peter (2002). The Biology of the Deep Ocean. Oxford University Press. pp. 192–195. ISBN 978-0-19-854956-7.
- ^ "Animal Circulatory Systems". Georgia Tech. Retrieved 10 February 2024.
- ^ Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia: Holt-Saunders International. pp. 316–327. ISBN 0-03-910284-X.
- ^ a b Graham, Jeffrey B.; Wegner, N.C. (2010). "6. Breathing air in water and in air: the air-breathing fishes". In Nilsson, Göran E. (ed.). Respiratory Physiology of Vertebrates. New York: Cambridge University Press. pp. 174–221. doi:10.1017/CBO9780511845178.007. ISBN 978-0-521-87854-8.
- ^ Armbruster, Jonathan W. (1998). "Modifications of the Digestive Tract for Holding Air in Loricariid and Scoloplacid Catfishes" (PDF). Copeia. 1998 (3): 663–675. doi:10.2307/1447796. JSTOR 1447796. Archived from the original (PDF) on 26 March 2009. Retrieved 25 June 2009.
- ^ "Digestive System". University of Tennessee. Retrieved 10 February 2024.
- ^ Burton, Derek; Burton, Margaret (21 December 2017). "Excretion". Oxford Scholarship Online. Oxford University Press. doi:10.1093/oso/9780198785552.003.0008.
- ^ Maetz, J. (20 August 1971). "Fish gills: mechanisms of salt transfer in fresh water and sea water". Philosophical Transactions of the Royal Society of London B. 262 (842): 209–249. Bibcode:1971RSPTB.262..209M. doi:10.1098/rstb.1971.0090. ISSN 0080-4622.
- ^ a b Helfman, Collette & Facey 1997, pp. 48–49.
- ^ Helfman, Collette & Facey 1997, p. 191.
- ^ Bleckmann, Horst; Zelick, Randy (1 March 2009). "Lateral line system of fish". Integrative Zoology. 4 (1): 13–25. doi:10.1111/j.1749-4877.2008.00131.x. PMID 21392273.
- ^ Godfrey-Smith, Peter (2020). "Kingfish". Metazoa. New York: Farrar, Straus and Giroux. ISBN 9780374207946.
- ^ a b Albert, J. S.; Crampton, W. G. (2006). "Electroreception and Electrogenesis". In Lutz, P. L. (ed.). The Physiology of Fishes. Boca Raton, Florida: CRC Press. pp. 429–470. ISBN 978-0-8493-2022-4.
- ^ a b c d Guthrie, D. M. (1986). "Role of Vision in Fish Behaviour". The Behaviour of Teleost Fishes. Boston, Massachusetts: Springer. pp. 75–113. doi:10.1007/978-1-4684-8261-4_4. ISBN 978-1-4684-8263-8.
- ^ Hawryshyn, Craig W. (2010). "Ultraviolet Polarization Vision and Visually Guided Behavior in Fishes". Brain, Behavior and Evolution. 75 (3): 186–194. doi:10.1159/000314275. ISSN 0006-8977. PMID 20733294.
- ^ Meyer-Rochow, V. Benno; Stewart, Duncan (1996). "Review of larval and postlarval eye ultrastructure in the lamprey (cyclostomata) with special emphasis on Geotria australis (gray)". Microscopy Research and Technique. 35 (6): 431–444. doi:10.1002/(SICI)1097-0029(19961215)35:6<431::AID-JEMT3>3.0.CO;2-L. PMID 9016447. S2CID 22940203.
- ^ Lamb, Trevor D.; Collin, Shaun P.; Pugh, Edward N. (2007). "Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup". Nature Reviews Neuroscience. 8 (12): 960–976. doi:10.1038/nrn2283. ISSN 1471-003X. PMC 3143066. PMID 18026166. See also Lamb et al.'s "The origin of the Vertebrate Eye", 2008.
- ^ Hawkins, A. D. (1981). "6. The Hearing Abilities of Fish". In Tavolga, William N.; Popper, Arthur N.; Fay, Richard R. (eds.). Hearing and Sound Communication in Fishes. Springer. pp. 109–138. ISBN 978-1-4615-7188-9.
- ^ Quinn, Thomas P. (1980). "Evidence for celestial and magnetic compass orientation in lake migrating sockeye salmon fry". Journal of Comparative Physiology A. 137 (3): 243–248. doi:10.1007/bf00657119. S2CID 44036559.
- ^ Taylor, P. B. (May 1986). "Experimental evidence for geomagnetic orientation in juvenile salmon, Oncorhynchus tschawytscha Walbaum". Journal of Fish Biology. 28 (5): 607–623. Bibcode:1986JFBio..28..607T. doi:10.1111/j.1095-8649.1986.tb05196.x.
- ^ Formicki, Krzysztof; Korzelecka-Orkisz, Agata; Tański, Adam (2019). "Magnetoreception in fish". Journal of Fish Biology. 95 (1): 73–91. Bibcode:2019JFBio..95...73F. doi:10.1111/jfb.13998. ISSN 0022-1112. PMID 31054161.
- ^ Hore, Peter J.; Mouritsen, Henrik (April 2022). "The Quantum Nature of Bird Migration". Scientific American: 24–29.
- ^ Ari, Csilla; D'Agostino, Dominic P. (1 May 2016). "Contingency checking and self-directed behaviors in giant manta rays: Do elasmobranchs have self-awareness?". Journal of Ethology. 34 (2): 167–174. doi:10.1007/s10164-016-0462-z. S2CID 254134775. Archived from the original on 17 March 2023. Retrieved 21 January 2023.
- ^ Kohda, Masanori; Hotta, Takashi; Takeyama, Tomohiro; Awata, Satoshi; Tanaka, Hirokazu; Asai, Jun-ya; Jordan, L. Alex (21 August 2018). "Cleaner wrasse pass the mark test. What are the implications for consciousness and self-awareness testing in animals?". PLOS Biology. 17 (2): 397067. bioRxiv 10.1101/397067. doi:10.1371/journal.pbio.3000021. PMC 6366756. PMID 30730878. S2CID 91375693.
- ^ Balcombe, Jonathan (1 May 2017). "Fishes Use Problem-Solving and Invent Tools". Scientific American. Archived from the original on 17 March 2023.
- ^ Laubu, Chloé; Louâpre, Philippe; Dechaume-Moncharmont, François-Xavier (2019). "Pair-bonding influences affective state in a monogamous fish species". Proceedings of the Royal Society B. 286 (1904). 20190760. doi:10.1098/rspb.2019.0760. PMC 6571461. PMID 31185864.
- ^ Sciences, Journal of Undergraduate Life. "Appropriate maze methodology to study learning in fish" (PDF). Archived from the original (PDF) on 6 July 2011. Retrieved 28 May 2009.
- ^ Woodruff, Michael (3 July 2020). "The face of the fish". Aeon. Retrieved 28 July 2024.
- ^ von der Emde, G. (15 May 1999). "Active electrolocation of objects in weakly electric fish". Journal of Experimental Biology. 202 (10): 1205–1215. doi:10.1242/jeb.202.10.1205. PMID 10210662.
- ^ Catania, Kenneth C. (20 October 2015). "Electric eels use high-voltage to track fast-moving prey". Nature Communications. 6: 8638. Bibcode:2015NatCo...6.8638C. doi:10.1038/ncomms9638. PMC 4667699. PMID 26485580.
- ^ a b Block, B.A.; Finnerty, JR (1993). "Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures" (PDF). Environmental Biology of Fishes. 40 (3): 283–302. doi:10.1007/BF00002518. S2CID 28644501. Archived from the original on 6 November 2020. Retrieved 1 October 2018.
- ^ Wegner, Nicholas C.; Snodgrass, Owyn E.; Dewar, Heidi; Hyde, John R. (15 May 2015). "Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus". Science. 348 (6236): 786–789. Bibcode:2015Sci...348..786W. doi:10.1126/science.aaa8902. ISSN 0036-8075. PMID 25977549. S2CID 17412022.
- ^ Goldman, K.J. (1997). "Regulation of body temperature in the white shark, Carcharodon carcharias". Journal of Comparative Physiology. B Biochemical Systemic and Environmental Physiology. 167 (6): 423–429. doi:10.1007/s003600050092. S2CID 28082417. Archived from the original on 6 April 2012. Retrieved 12 October 2011.
- ^ Carey, F.G.; Lawson, K.D. (February 1973). "Temperature regulation in free-swimming bluefin tuna". Comparative Biochemistry and Physiology A. 44 (2): 375–392. doi:10.1016/0300-9629(73)90490-8. PMID 4145757.
- ^ Guimaraes-Cruz, Rodrigo J.; dos Santos, José E.; Santos, Gilmar B. (July–September 2005). "Gonadal structure and gametogenesis of Loricaria lentiginosa Isbrücker (Pisces, Teleostei, Siluriformes)". Rev. Bras. Zool. 22 (3): 556–564. doi:10.1590/S0101-81752005000300005. ISSN 0101-8175.
- ^ Brito, M.F.G.; Bazzoli, N. (2003). "Reproduction of the surubim catfish (Pisces, Pimelodidae) in the São Francisco River, Pirapora Region, Minas Gerais, Brazil". Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 55 (5): 624–633. doi:10.1590/S0102-09352003000500018. ISSN 0102-0935.
- ^ Scott, Peter (1997). Livebearing Fishes. Tetra Press. p. 13. ISBN 1-56465-193-2.
- ^ a b c Miller, Bruce; Kendall, Arthur W. (2009). "1. Fish Reproduction" (PDF). Early Life History of Marine Fishes. University of California Press. pp. 11–37. ISBN 9780520249721. Archived (PDF) from the original on 7 March 2016.
- ^ a b Dey A, Flajšhans M, Pšenička M, Gazo I. DNA repair genes play a variety of roles in the development of fish embryos. Front Cell Dev Biol. 2023 Mar 1;11:1119229. doi: 10.3389/fcell.2023.1119229. PMID 36936683; PMCID: PMC10014602
- ^ Canedo A, Rocha TL. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: Historical review, current status and trends. Sci Total Environ. 2021 Mar 25;762:144084. doi: 10.1016/j.scitotenv.2020.144084. Epub 2020 Dec 14. PMID 33383303
- ^ Helfman, Collette & Facey 1997, pp. 95–96.
- ^ a b c Flajnik, M. F.; Kasahara, M. (2010). "Origin and evolution of the adaptive immune system: genetic events and selective pressures". Nature Reviews Genetics. 11 (1): 47–59. doi:10.1038/nrg2703. PMC 3805090. PMID 19997068.
- ^ Zapata, A.G.; Chiba, A.; Vara, A. (1996). "Cells and tissues of the immune system of fish". In Iwama, G. Iwama; Nakanishi, T. (eds.). The Fish Immune System: Organism, Pathogen and Environment. Fish Immunology. New York: Academic Press. pp. 1–55.
- ^ Chilmonczyk, S. (1992). "The thymus in fish: development and possible function in the immune response". Annual Review of Fish Diseases. 2: 181–200. doi:10.1016/0959-8030(92)90063-4.
- ^ Hansen, J.D.; Zapata, A.G. (1998). "Lymphocyte development in fish and amphibians". Immunological Reviews. 166: 199–220. doi:10.1111/j.1600-065x.1998.tb01264.x. PMID 9914914. S2CID 7965762.
- ^ a b Pitcher, Tony J. (1986). "12. Functions of Shoaling Behaviour in Teleosts". The Behaviour of Teleost Fishes. Springer. pp. 294–337. doi:10.1007/978-1-4684-8261-4_12. ISBN 978-1-4684-8263-8.
- ^ Helfman, Collette & Facey 1997, p. 375.
- ^ Gjøsæter, H. (1998). "The population biology and exploitation of capelin (Mallotus villosus) in the Barents Sea". Sarsia. 83 (6): 453–496. doi:10.1080/00364827.1998.10420445.
- ^ Weinmann, S.R.; Black, A.N.; Richter, M.L.; Itzkowitz, M.; Burger, R.M. (February 2017). "Territorial vocalization in sympatric damselfish: acoustic characteristics and intruder discrimination". Bioacoustics. 27 (1): 87–102. doi:10.1080/09524622.2017.1286263. S2CID 89625932.
- ^ a b Bertucci, F.; Ruppé, L.; Wassenbergh, S.V.; Compère, P.; Parmentier, E. (29 October 2014). "New Insights into the Role of the Pharyngeal Jaw Apparatus in the Sound-Producing Mechanism of Haemulon Flavolineatum (Haemulidae)". Journal of Experimental Biology. 217 (21): 3862–3869. doi:10.1242/jeb.109025. hdl:10067/1197840151162165141. PMID 25355850.
- ^ Colson, D.J.; Patek, S.N.; Brainerd, E.L.; Lewis, S.M. (February 1998). "Sound production during feeding in Hippocampus seahorses (Syngnathidae)". Environmental Biology of Fishes. 51 (2): 221–229. Bibcode:1998EnvBF..51..221C. doi:10.1023/A:1007434714122. S2CID 207648816.
- ^ Oliveira, T.P.R.; Ladich, F.; Abed-Navandi, D.; Souto, A.S.; Rosa, I.L. (26 June 2014). "Sounds produced by the longsnout seahorse: a study of their structure and functions". Journal of Zoology. 294 (2): 114–121. doi:10.1111/jzo.12160.
- ^ a b Fine, L.F.; King, C.B.; Cameron, T.M. (16 October 2009). "Acoustical properties of the swimbladder in the oyster toadfish Opsanus tau". Journal of Experimental Biology. 212 (21): 3542–3552. doi:10.1242/jeb.033423. PMC 2762879. PMID 19837896.
- ^ Fine, M.L.; Waybright, T.D. (15 October 2015). "Grunt variation in the oyster toadfish Opsanus tau:effect of size and sex". PeerJ. 3 (1330): e1330. doi:10.7717/peerj.1330. PMC 4662586. PMID 26623178.
- ^ a b c Ricci, S.W.; Bohnenstiehl, D. R.; Eggleston, D.B.; Kellogg, M.L.; Lyon, R.P. (8 August 2017). "Oyster toadfish (Opsanus tau) boatwhistle call detection and patterns within a large-scale oyster restoration site". PLOS ONE. 12 (8): e0182757. Bibcode:2017PLoSO..1282757R. doi:10.1371/journal.pone.0182757. PMC 5549733. PMID 28792543.
- ^ Skoglund, C.R. (1 August 1961). "Functional analysis of swimbladder muscles engaged in sound productivity of the toadfish". Journal of Cell Biology. 10 (4): 187–200. doi:10.1083/jcb.10.4.187. PMC 2225107. PMID 19866593.
- ^ Parmentier, E.; Tock, J.; Falguière, J.C.; Beauchaud, M. (22 May 2014). "Sound production in Sciaenops ocellatus: Preliminary study for the development of acoustic cues in aquaculture" (PDF). Aquaculture. 432: 204–211. Bibcode:2014Aquac.432..204P. doi:10.1016/j.aquaculture.2014.05.017. Archived (PDF) from the original on 3 June 2021. Retrieved 21 January 2019.
- ^ "Search for 'Fishes' (Global, CR-Critically Endangered, En-Endangered, Species)". Retrieved 27 February 2024.
- ^ Sobel, J. (1996). "Gadus morhua". IUCN Red List of Threatened Species. 1996: e.T8784A12931575. doi:10.2305/IUCN.UK.1996.RLTS.T8784A12931575.en. Retrieved 11 November 2021.
- ^ NatureServe (2014). "Cyprinodon diabolis". IUCN Red List of Threatened Species. 2014: e.T6149A15362335. doi:10.2305/IUCN.UK.2014-3.RLTS.T6149A15362335.en. Retrieved 11 November 2021.
- ^ Musick, J.A. (2000). "Latimeria chalumnae". IUCN Red List of Threatened Species. 2000: e.T11375A3274618. doi:10.2305/IUCN.UK.2000.RLTS.T11375A3274618.en. Retrieved 11 November 2021.
- ^ Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; et al. (2019). "Carcharodon carcharias". IUCN Red List of Threatened Species. 2019: e.T3855A2878674. Retrieved 19 December 2019.
- ^ Helfman, Collette & Facey 1997, pp. 449–450.
- ^ a b Hamilton, Lawrence C.; Butler, M. J. (January 2001). "Outport adaptations: Social indicators through Newfoundland's Cod crisis". Human Ecology Review. 8 (2): 1–11.
- ^ The State of World Fisheries and Aquaculture 2020. Food and Agriculture Organization. 2020. p. 54. doi:10.4060/ca9229en. hdl:10535/3776. ISBN 978-92-5-132692-3. S2CID 242949831.
- ^ "Call to halt cod 'over-fishing'". BBC News. 5 January 2007. Archived from the original on 17 January 2007. Retrieved 18 January 2006.
- ^ "Tuna groups tackle overfishing". BBC News. 26 January 2007. Archived from the original on 21 January 2009. Retrieved 18 January 2006.
- ^ Helfman, Collette & Facey 1997, p. 462.
- ^ "UK 'must shield fishing industry'". BBC News. 3 November 2006. Archived from the original on 30 November 2006. Retrieved 18 January 2006.
- ^ "EU fish quota deal hammered out". BBC News. 21 December 2006. Archived from the original on 26 December 2006. Retrieved 18 January 2006.
- ^ "Ocean study predicts the collapse of all seafood fisheries by 2050". phys.org. Archived from the original on 15 March 2007. Retrieved 13 January 2006.
- ^ "Atlantic bluefin tuna could soon be commercially extinct". WWF. Archived from the original on 30 April 2007. Retrieved 18 January 2006.
- ^ Helfman, Collette & Facey 1997, p. 463.
- ^ Elvira, Benigno (1995). "Conservation status of endemic freshwater fish in Spain". Biological Conservation. 72 (2). Elsevier: 129–136. Bibcode:1995BCons..72..129E. doi:10.1016/0006-3207(94)00076-3. ISSN 0006-3207.
- ^ Jackson, Donald C.; Marmulla, Gerd (2001). The influence of dams on river fisheries (PDF). Vol. Technical paper 419. FAO Fisheries. pp. 1–44.
- ^ Duplisea, Daniel E.; Frisk, Michael G.; Trenkel, Verena M. (28 November 2016). "Extinction Debt and Colonizer Credit on a Habitat Perturbed Fishing Bank". PLOS ONE. 11 (11). Public Library of Science (PLoS): e0166409. Bibcode:2016PLoSO..1166409D. doi:10.1371/journal.pone.0166409. ISSN 1932-6203. PMC 5125594. PMID 27893775.
- ^ a b Lovell, Sabrina J.; Stone, Susan F.; Fernandez, Linda (2006). "The economic impacts of aquatic invasive species: a review of the literature". Agricultural and Resource Economics Review. 35 (1): 195–208. doi:10.1017/S1068280500010157.
- ^ Knowler, D.; Barbier, E.B. (2000). "The Economics of an Invading Species: a Theoretical Model and Case Study Application". In Perrings, C.; Williamson, M.; Dalmazzone, S. (eds.). The Economics of Biological Invasions. Cheltenham: Edward Elgar. pp. 70–93.
- ^ Atlas of Exotic Fishes in the Mediterranean Sea. 2nd Edition. 2021. (F. Briand Ed.) CIESM Publishers, Paris, Monaco 366 p.[1]
- ^ Golani, Daniel (1998). "Impact of Red Sea fish migrants through the Suez Canal on the aquatic environment of the Eastern Mediterranean". Bulletin Series Yale School of Forestry and Environmental Studies (103): 375–387.
- ^ Coulter, George W.; Allanson, Brian R.; Bruton, Michael N.; Greenwood, P. Humphry; Hart, Robert C.; Jackson, Peter B. N.; Ribbink, Anthony J. (1986). "Unique qualities and special problems of the African Great Lakes". Environmental Biology of Fishes. 17 (3). Springer Science and Business Media: 161–183. Bibcode:1986EnvBF..17..161C. doi:10.1007/bf00698196. ISSN 0378-1909.
- ^ Achieng, A. P. (1990). "The impact of the introduction of the Nile Perch, Lates niloticus (L.), on the fisheries of Lake Victoria". Journal of Fish Biology. 37, Suppl. A: 17–23. Bibcode:1990JFBio..37S..17A. doi:10.1111/j.1095-8649.1990.tb05016.x.
- ^ Spalding, Mark (11 July 2013). "Sustainable Ancient Aquaculture". National Geographic. Archived from the original on 18 May 2015. Retrieved 13 August 2015.
- ^ a b Helfman, Gene S. (2007). Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources. Island Press. p. 11. ISBN 978-1-59726-760-1.
- ^ "EDF Seafood Selector: Fish Choices that are Good for You and the Oceans". Environmental Defense Fund. Retrieved 21 January 2024.
- ^ FAO (2022). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome: Food and Agriculture Organization. doi:10.4060/cc0461en. hdl:10535/3776. ISBN 978-92-5-136364-5.
- ^ Beard, T. Douglas, ed. (2011). The Angler in the Environment: Social, Economic, Biological, and Ethical Dimensions. Bethesda, Maryland: American Fisheries Society. p. 365. ISBN 978-1-934874-24-0.
- ^ Hickley, Phil; Tompkins, Helena, eds. (1998). Recreational Fisheries: Social, Economic and Management Aspects. Wiley-Blackwell. p. 328. ISBN 978-0-852-38248-6.
- ^ a b c d e Black, Jeremy; Green, Anthony (1992). Gods, Demons and Symbols of Ancient Mesopotamia: An Illustrated Dictionary. The British Museum Press. pp. 82–83. ISBN 978-0-7141-1705-8. Archived from the original on 20 February 2018.
- ^ a b c Hyde, Walter Woodburn (2008) [1946]. Paganism to Christianity in the Roman Empire. Eugene, Oregon: Wipf and Stock Publishers. pp. 57–58. ISBN 978-1-60608-349-9. Archived from the original on 17 March 2023. Retrieved 12 December 2020.
- ^ Sherwood, Yvonne (2000), A Biblical Text and Its Afterlives: The Survival of Jonah in Western Culture, Cambridge University Press, pp. 1–8, ISBN 978-0-521-79561-6, archived from the original on 17 March 2023, retrieved 12 December 2020
- ^ Coffman, Elesha (8 August 2008). "What is the origin of the Christian fish symbol?". Christianity Today. Archived from the original on 30 January 2016. Retrieved 13 August 2015.
- ^ "'Ngārara – reptiles, Page 2. From sea to land', Te Ara - the Encyclopedia of New Zealand". Bradford Haami. Retrieved 4 May 2018.
- ^ Thrum, Thomas (1907). Hawaiian Folk Tales. A. C. McClurg. p. 86.
- ^ Bandyopadhyaya, Jayantanuja (2007). Class and Religion in Ancient India. Anthem Press. p. 136. ISBN 978-1-84331-332-8. Archived from the original on 8 October 2022. Retrieved 10 July 2022.
- ^ Ovid Fasti 2.457ff
- ^ Moyle, Peter B.; Moyle, Marilyn A. (May 1991). "Introduction to fish imagery in art". Environmental Biology of Fishes. 31 (1): 5–23. Bibcode:1991EnvBF..31....5M. doi:10.1007/bf00002153. S2CID 33458630.
- ^ Tidwell, Christy (2009). "'Fish Are Just like People, Only Flakier': Environmental Practice and Theory in Finding Nemo". Americana: The Journal of American Popular Culture (8).
- ^ Durga, P.; Sai, Kanaka (2017). "Nature of Existential Struggle in The Old Man and the Sea". Journal of English Language and Literature JOELL. 4 (4): 19–21.
- ^ Alabaster, Jay (2023). "The Goofy Great White: Jaws and Our Love for an Apex Predator". In Jackson, Kathy Merlock; Simpson, Philip L. (eds.). This shark, swallow you whole": Essays on the Cultural Influence of Jaws. McFarland. pp. 124–. ISBN 978-1-4766-7745-3.
- ^ Zollinger, Sue Anne (3 July 2009). "Piranha – Ferocious Fighter or Scavenging Softie?". A Moment of Science. Indiana Public Media. Archived from the original on 17 October 2015. Retrieved 1 November 2015.
Sources
- Benton, M. J. (2005). Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. ISBN 978-140514449-0.
- Helfman, G.; Collette, B.; Facey, D. (1997). The Diversity of Fishes (1st ed.). Wiley-Blackwell. ISBN 978-0-86542-256-8.
- Nelson, Joseph S. (2016). "Taxonomic Diversity". Fishes of the World. John Wiley & Sons. ISBN 978-1-118-34233-6.
Further reading
- Eschmeyer, William N.; Fong, Jon David (2013). "Catalog of Fishes". California Academy of Sciences. Archived from the original on 21 November 2018. Retrieved 28 February 2013.
- Helfman, G.; Collette, B.; Facey, D.; Bowen, B. (2009). The Diversity of Fishes: Biology, Evolution, and Ecology (2nd ed.). Wiley-Blackwell. ISBN 978-1-4051-2494-2. Archived from the original on 26 August 2021. Retrieved 26 January 2010.
- Moyle, Peter B. (1993) Fish: An Enthusiast's Guide Archived 17 March 2023 at the Wayback Machine University of California Press. ISBN 978-0-520-91665-4 – good lay text.
- Moyle, Peter B.; Cech, Joseph J. (2003). Fishes, An Introduction to Ichthyology (5th ed.). Benjamin Cummings. ISBN 978-0-13-100847-2.
- Scales, Helen (2018). Eye of the shoal: A Fishwatcher's Guide to Life, the Ocean and Everything. Bloomsbury Sigma. ISBN 978-1-4729-3684-4.
- Shubin, Neil (2009). Your inner fish: A journey into the 3.5 billion year history of the human body. Vintage Books. ISBN 978-0-307-27745-9. Archived from the original on 17 March 2023. Retrieved 15 December 2015. UCTV interview Archived 14 January 2021 at the Wayback Machine
External links
- ANGFA – Illustrated database of freshwater fishes of Australia and New Guinea
- FishBase online – Comprehensive database with information on over 29,000 fish species
- Fisheries and Illinois Aquaculture Center – Data outlet for fisheries and aquaculture research center in the central US at archive.today (archived 15 December 2012)
- The Native Fish Conservancy – Conservation and study of North American freshwater fishes at the Wayback Machine (archived 12 March 2008)
- United Nation – Fisheries and Aquaculture Department: Fish and seafood utilization





























